Revolutionizing Our Understanding of Cell Division: The Surprising Role of CENP-E
In an unexpected twist in the world of cell biology, scientists at Zagreb’s Ruđer Bošković Institute (RBI) have upended long-held beliefs about a crucial protein known as CENP-E. For decades, researchers thought of CENP-E as a tiny motor pulling chromosomes toward the center of a dividing cell. But new findings suggest it plays a much different, yet equally vital, role in keeping everything organized during cell division.
A Shift in Perspective
Picture a busy road during rush hour, where every car must find its lane and position perfectly to avoid chaos. In the same way, chromosomes need precise placement within a cell before it can divide. The discovery made by Dr. Kruno Vukušić and Professor Iva Tolić at RBI reveals that CENP-E doesn’t act like a motor pulling chromosomes around; instead, it’s more like a traffic signal ensuring that everything gets attached properly before the cell splits in two.
This revelation goes beyond merely rephrasing a scientific mantra—it shakes the foundation of our understanding of cell division and has significant implications for studying diseases like cancer.
Why the Right Connections Matter
Every second, billions of cells are busily dividing, replicating a staggering three billion DNA letters. It’s a meticulous process, and even a single misplaced chromosome can have dire consequences, leading to issues like infertility, developmental disorders, and cancer. Indeed, the stakes are high, and cell division leaves little room for error.
For years, scientists clung to the notion that CENP-E was the muscle driving chromosomes into place. This theory, while neat and straightforward, was ultimately misleading. The RBI researchers have uncovered that while CENP-E is essential, its true function lies in establishing the first connections between chromosomes and the cell’s internal tracks—critical for guiding them to their rightful positions.
Unpacking CENP-E’s Role
To understand CENP-E’s newly defined role, imagine a city grappling with heavy traffic. Cars—representing chromosomes—are waiting to merge onto a busy interstate. If the traffic signals (CENP-E, in this analogy) aren’t functioning correctly, neither the cars nor the system itself can flow smoothly.
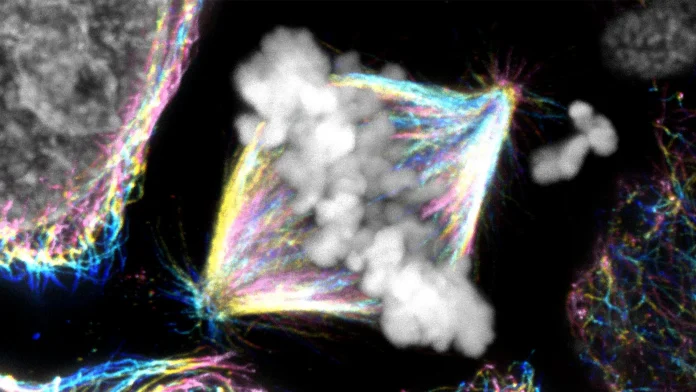
In this case, the cell’s “tracks” are microtubules, the structures that guide chromosomes through the division process. Instead of just pulling these chromosomes along, CENP-E acts as a connector, stabilizing the interactions between chromosomes and microtubules. Without this stable attachment, chromosomes aren’t able to advance, much like vehicles stalled far from the exit ramp.
The Science Behind the Signals
But what causes some chromosomes to hesitate at the cell’s edges? That’s where another cast of characters comes into play: the Aurora kinases, proteins that function like strict traffic lights, sending signals to halt chromosomes from making premature connections. While this system helps prevent mistakes, it can sometimes hold chromosomes back too much. CENP-E, in this role, helps regulate these signals, allowing proper connections to form without unnecessary delays.
“It’s about creating the right conditions for everything to run smoothly,” affirms Professor Tolić. CENP-E’s primary function is to ensure those early, crucial attachments are secure, paving the way for orderly division.
A Major Paradigm Shift
For almost two decades, textbooks described CENP-E as the engine of chromosome movement. The work from the researchers in Zagreb flips this narrative on its head. By demonstrating that CENP-E’s primary contribution is stabilizing the initial attachments rather than merely moving chromosomes, they unveil a more nuanced understanding of cell division dynamics.
This transition from a force-based approach to one focused on careful timing and regulation is a game changer, not just for academic textbooks but also for medical science. Why does this matter? Well, errors in chromosome segregation are a hallmark of various cancers, with tumor cells often exhibiting duplicated or missing chromosome segments. By honing in on CENP-E’s regulatory role, researchers are lifting the veil on a potential weak spot in dividing cells, perhaps pointing toward innovative therapies that can correct these dangerous malfunctions.
Implications for Human Health
For those not immersed in the science, the nuances may seem subtle. But in the world of cell biology, these tiny adjustments can unearth monumental truths. By connecting CENP-E’s regulatory role to the activities of Aurora kinases, the researchers have bridged two previously distinct processes. This holistic understanding opens new avenues for diagnostics and potential therapies for diseases that wreak havoc on the body’s natural order.
Dr. Vukušić paints a hopeful picture: “It’s not just about rewriting a model; it’s about pinpointing mechanisms that directly relate to diseases.” With this clarity, the chance to develop targeted interventions becomes all that much brighter.
Funding and Collaboration
The ambitious project received significant backing through various grants, including monetary support from the European Research Council’s Synergy Grant and the Croatian Science Foundation. The team at RBI also benefited from advanced computing resources at the University of Zagreb, highlighting the increasingly collaborative nature of modern biology that transcends geographical borders and disciplines.
“Today’s biology isn’t just about lab equipment,” Tolić emphasizes. “It thrives on computation and interdisciplinary collaboration.”
Finding Order in Cellular Chaos
At its core, the research highlights how cells manage to maintain order amidst seemingly endless chaos. With trillions of cell divisions occurring within our bodies every day, each one must strive against the pull of disorder. This new understanding from Zagreb helps peel back one more layer of complexity, showcasing a regulatory strategy that maintains cellular integrity in the face of potential pandemonium.
As Tolić notes, “By revealing how these microscopic regulators work together, we deepen our understanding of biology and enhance our potential to address diseases at their root.”
A Closing Thought
This groundbreaking research doesn’t just redefine our understanding of CENP-E; it reshapes our perception of cell division as a whole. It serves as a powerful reminder of how much we still have to learn about life at the microscopic level. The path forward is illuminated not just by the laughter and joy of cell division but also by the darkness that can come from its mistakes. Recognizing these connections is the first step in paving the way for future therapies and innovations that could one day alleviate the personal and collective burdens of diseases like cancer.
What does this mean for all of us? It means paying closer attention to the intricate dance of our cells and the delicate balances that shape our health. Perhaps one day, those balances will be easier to maintain, allowing us all to thrive.